Pascal’s Law is a fundamental law of gas hydrodynamics formulated by the French mathematician and physicist Blaise Pascal in the 17th century. This law describes how changes in pressure affect gases in closed systems and is one of the fundamental properties of gases.
Formulation of the law
Pascal’s Law is formulated as follows:
Liquids and gases transmit pressure applied to them equally in all directions.
This means that if pressure is applied to a gas at one point in a container (or vessel), this pressure is evenly distributed in all directions, including the inner walls of the container and the rest of the gas inside the container.
In the case of uniform distribution of pressure, the pressure on all areas of the surface is the same and numerically equals the pressure force acting perpendicular to the surface divided by the area of that surface.
Pressure, denoted as “p,” is defined as a scalar physical quantity equal to the magnitude of the pressure force acting perpendicular to a surface divided by the area of that surface:
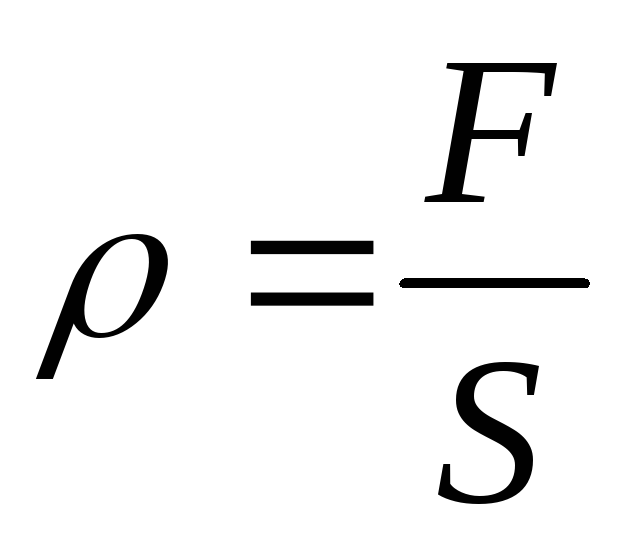
In the International System of Units (SI), the unit of pressure is the pascal (Pa):
1 Pa = 1 N/m².
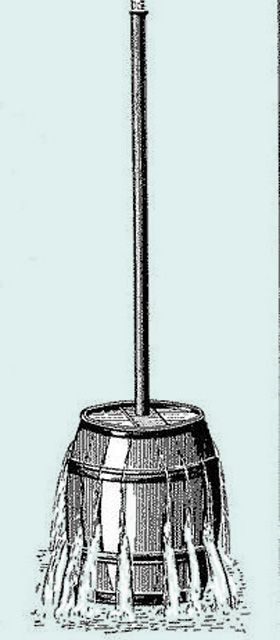
An illustration of this law is Pascal’s “ruptured barrel” experiment conducted in 1646. He inserted a tube that was 10 meters long into a barrel filled with water. When Pascal added water to the tube, the increased pressure inside the barrel caused it to rupture into separate pieces.