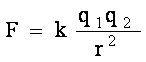Coulomb’s law states that the force between two charged particles is directly proportional to the product of their charges and inversely proportional to the square of the distance between them. Mathematically, this can be expressed as:
where
F is the force between the two charged particles,
q1 and q2 are the magnitudes of their charges,
r is the distance between them,
k is a constant of proportionality known as the Coulomb constant.
The Coulomb constant is a fundamental physical constant that relates the units of charge, distance, and force. Its value is approximately (k ≈ 8.988×109 N⋅m2⋅C−2), where N is the unit of force, m is the unit of distance, and C is the unit of electric charge.
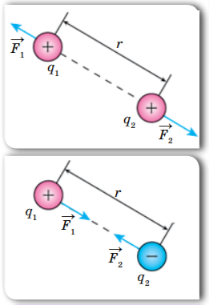
Coulomb’s Law applies to any type of charged particle, whether it be a single electron, a proton, or a larger object with a net charge. The force between two particles can be either attractive or repulsive, depending on the sign of their charges. Like charges (i.e. two positively charged particles or two negatively charged particles) will repel each other, while opposite charges will attract.
The inverse square relationship between force and distance means that the force decreases rapidly as the distance between the charged particles increases. For example, if the distance between two charged particles is doubled, the force between them will decrease by a factor of four. This relationship is a key aspect of Coulomb’s Law and helps to explain why electric fields weaken rapidly with distance.
Using Coulomb’s law
Coulomb’s Law is used in a wide range of applications, including electrical engineering, chemistry, and physics. It is particularly important in the design and operation of electrical circuits and devices, where it is used to calculate the forces and interactions between charged particles. Coulomb’s Law also plays a key role in understanding the behavior of atoms and molecules, where the interactions between charged particles help to determine chemical properties and reactions.
In conclusion, Coulomb’s Law is a fundamental principle of electrostatics that describes the force between two charged particles. It is a simple yet powerful formula that has wide-ranging applications in science and engineering, and has played a critical role in our understanding of the natural world.