Electron (Greek Ηλεκτρόνιο) is an elementary particle carrying a negative electric charge. It is one of the fundamental constituents of atoms, alongside protons and neutrons.
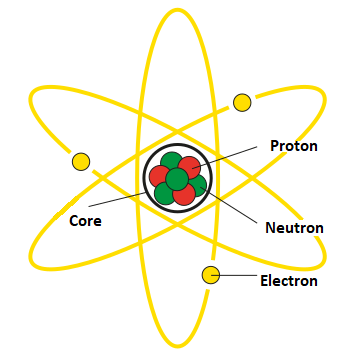
The electron has a small mass compared to protons and neutrons and orbits the atomic nucleus in electron clouds. It possesses an electric charge (−e = −1.6021892(46)×10−19 C) and a mass (9.109554(906)×10−31 kg).
Electrons play a crucial role in electronics, being responsible for conducting electric current in wires. They also participate in chemical reactions, facilitating the exchange processes between atoms, leading to the formation of chemical bonds between them.
Typically, the electron is represented in formulas by the symbol e–.
Discovery of the Electron
The history of the discovery of the electron begins in the late 19th century when scientists were conducting research on electromagnetism and electricity. However, prior to the discovery of the electron, scientists believed that the atom was the smallest indivisible unit of matter.
In the first half of the 19th century, physicists conducted research on electric currents and electromagnetism, which had been studied since the time of Benjamin Franklin and Michael Faraday. In 1864, James Clerk Maxwell developed a mathematical model of the electromagnetic field, but the theory couldn’t explain how electromagnetic waves propagate through space.
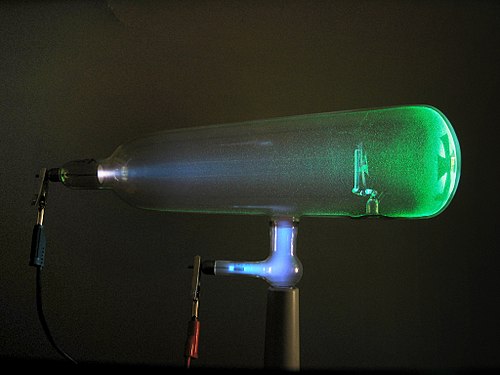
In 1873, William Crookes performed experiments on gas discharge in a vacuum tube and found that electric current could pass through a vacuum. Crookes also observed that if an electric current passed through a vacuum tube, a glow appeared on the tube’s walls. In 1876, Eugen Goldstein conducted similar experiments and discovered new rays, which he called cathode rays. Crookes’ Electron Tube.
In 1895, Wilhelm Röntgen discovered X-rays, which were found to penetrate through materials that absorb light. Röntgen was able to produce images of his wife’s hand skeleton, which revealed the bone structure. This discovery led to the development of a new field in medicine – radiology.
In 1897, J.J. Thomson confirmed Goldstein’s hypothesis that cathode rays consist of negatively charged particles. Thomson conducted experiments with cathode rays emitted in different directions and found that they carried a negative charge.
Later the same year, British physicist Joseph John Thomson performed an experiment that revealed the existence of the electron. Thomson used a vacuum tube similar to the one Goldstein used. The tube consisted of a glass bulb with two electrodes: a cathode and an anode. When an electric current passed through the tube, Thomson observed a stream of electrons emitted from the cathode and moving towards the anode.
Thomson also discovered that electrons had a small mass and a negative charge, becoming the first scientist to identify a negatively charged elementary particle. He was also able to determine the charge-to-mass ratio of the electron.
The discovery of the electron opened the doors to a new era in science, where electrons became key particles in understanding the structure of atoms and molecules. Electrons form the basis of electric current and electronics. Electronic devices such as televisions, computers, and phones utilize the properties of electrons to transmit and process information.
In the years following the discovery of the electron, extensive research has been conducted on the properties of electrons.