The atom is the basic unit of matter, consisting of a nucleus of protons and neutrons, surrounded by electrons that orbit the nucleus in shells or energy levels.
The history of the models of the atom is the story of how scientists have attempted to understand the structure of the atom, from the early days of ancient Greek philosophy to the present day.
This article will explore the history of the models of the atom, beginning with the ancient Greeks and continuing through to the present day. We will examine the different models of the atom that have been proposed, including the Thomson model, Rutherford model, Bohr model, and quantum mechanical model. We will also explore the structure of the atom, including the properties of protons, neutrons, and electrons, and the various forces that hold the atom together.
History of Models of the Atom
The idea of the atom can be traced back to the ancient Greek philosophers Democritus and Leucippus, who proposed that matter was composed of tiny, indivisible particles called atoms. However, this idea did not gain widespread acceptance until the 19th century, when scientists began to conduct experiments that suggested the existence of atoms.
One of the first models of the atom was proposed by John Dalton in the early 19th century. Dalton proposed that atoms were solid, indivisible spheres, and that different elements were composed of different types of atoms. This model was a major step forward in understanding the structure of matter, but it was later shown to be incomplete.
Thomson’s model of the atom
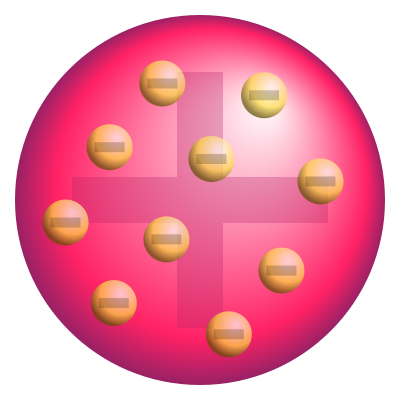
In 1897, J.J. Thomson discovered the electron, a negatively charged particle that is found outside the nucleus of the atom. Thomson proposed that atoms were composed of a positive sphere of matter, with negatively charged electrons embedded within it. This model became known as the Thomson model of the atom, and it was the first to suggest that the atom was not a solid, indivisible sphere.
Rutherford’s model of the atom
In 1911, Ernest Rutherford conducted the famous gold foil experiment, which led to the discovery of the nucleus. Rutherford proposed that atoms consisted of a small, dense, positively charged nucleus, with electrons orbiting the nucleus at a distance. This model became known as the Rutherford model of the atom, and it was a major advance in our understanding of the structure of the atom.
In 1913, Niels Bohr proposed a modified version of the Rutherford model, in which he suggested that electrons orbit the nucleus in discrete energy levels or shells. Bohr’s model explained many of the spectral lines observed in the emission and absorption spectra of atoms, and it was a significant advance in our understanding of atomic structure.
In the 1920s and 1930s, a new model of the atom emerged, based on the principles of quantum mechanics. This model, which is sometimes called the quantum mechanical model or the wave-mechanical model, suggests that electrons do not orbit the nucleus in a fixed path, but rather exist in a probability cloud around the nucleus. This model has become the most widely accepted model of the atom, and it has led to many advances in our understanding of the behavior of atoms and molecules.
The Structure of the Atom
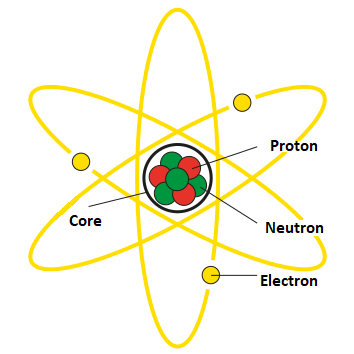
The atom is composed of three types of particles: protons, neutrons, and electrons. Protons and neutrons are found in the nucleus of the atom, while electrons orbit the nucleus in shells or energy levels.
Protons are positively charged particles, with a mass of approximately 1 atomic mass unit (amu). Neutrons are neutral particles, with a mass of approximately 1 amu. Electrons are negatively charged particles, with a mass of approximately 1/1836 amu.
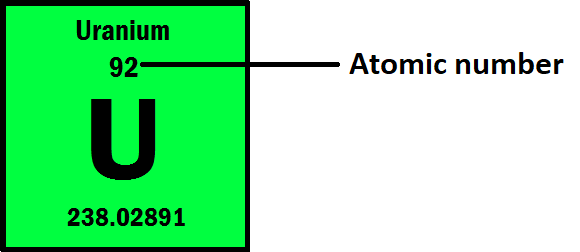
The number of protons in the nucleus of an atom is called the atomic number, and it determines the identity of the element.
For example, an atom with six protons in its nucleus is carbon, while an atom with eight protons is oxygen. The total number of protons and neutrons in the nucleus of an atom is called the mass number.
The electrons in an atom are arranged in shells or energy levels, which are located at varying distances from the nucleus. The first shell, which is closest to the nucleus, can hold up to 2 electrons, while the second shell can hold up to 8 electrons, and so on. The number of electrons in the outermost shell of an atom is called the valence electrons, and it determines the chemical properties of the element.
The behavior of atoms is governed by four fundamental forces: the electromagnetic force, the strong nuclear force, the weak nuclear force, and gravity. The electromagnetic force is responsible for holding the electrons in their orbits around the nucleus, while the strong nuclear force holds the protons and neutrons together in the nucleus. The weak nuclear force is responsible for some types of radioactive decay, while gravity is a much weaker force that acts on all matter.
The size of an atom
The size of an atom can be described in terms of its atomic radius, which is the distance from the center of the nucleus to the outermost shell of electrons. The size of an atom can vary depending on its atomic number (the number of protons in the nucleus) and the number of electrons in its outermost shell.
Atoms can range in size from about 0.1 to 0.5 nanometers (nm), or 1 to 5 angstroms (Å) in diameter. For example, the atomic radius of a hydrogen atom is about 0.053 nm (or 0.53 Å), while the atomic radius of a uranium atom is about 0.156 nm (or 1.56 Å).
It is important to note that the size of an atom is not a fixed value, but rather a range of values that depends on how the atom is bound in a molecule or solid. In addition, the size of an atom can be influenced by external factors, such as temperature and pressure.
Atomic mass
Atomic mass refers to the mass of an atom, typically expressed in atomic mass units (amu). The atomic mass of an atom is primarily determined by the number of protons and neutrons in its nucleus.
The atomic mass unit is defined as 1/12th the mass of a carbon-12 atom. Therefore, the atomic mass of an atom is calculated by adding the number of protons and neutrons in the nucleus. For example, a carbon-12 atom has 6 protons and 6 neutrons, giving it an atomic mass of 12 amu.
It’s important to note that the atomic mass listed on the periodic table for each element is an average of the masses of all the naturally occurring isotopes of that element, taking into account the abundance of each isotope. This is why some atomic masses on the periodic table may be decimal numbers instead of whole numbers.
Conclusion
The history of the models of the atom is the story of how scientists have attempted to understand the structure of the atom, from the early days of ancient Greek philosophy to the present day. The different models of the atom that have been proposed, including the Thomson model, Rutherford model, Bohr model, and quantum mechanical model, have each contributed to our understanding of atomic structure and behavior.
Today, the quantum mechanical model is the most widely accepted model of the atom, and it has led to many advances in our understanding of the behavior of atoms and molecules. The structure of the atom, including the properties of protons, neutrons, and electrons, and the various forces that hold the atom together, is a fundamental concept in chemistry and physics, and it has important applications in fields such as materials science, medicine, and energy production.