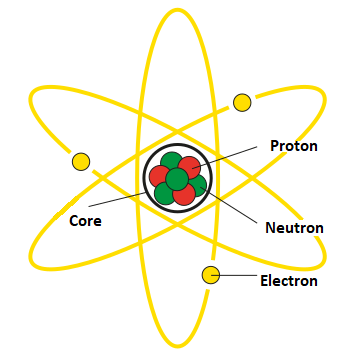
Proton – is an elementary particle that constitutes the nucleus of an atom along with neutrons. It has a positive electric charge and is symbolized by the letter “p.”
Protons are responsible for the chemical properties of elements as they determine the quantity and distribution of electrons in an atom.
Historically, the proton was discovered in 1917 by the American physicist Ernest Rutherford while conducting experiments on the scattering of alpha particles on metal foil screens. He noticed that most alpha particles passed through the screen, but some were deflected at large angles. This indicated the presence of positively charged particles in the atom’s nucleus, which were named protons.
A proton has a mass of approximately 1.007276 atomic mass units and a charge of +1 elementary charge. It is a stable particle and does not undergo radioactive decay.
Description and structure
The description of a proton from the perspective of modern physics is based on the Standard Model of elementary particles, which describes a proton as a combination of quarks. Quarks are fundamental particles that form the basis of more complex particles.

A proton is composed of three quarks: two “up” quarks and one “down” quark, held together by gluons.
Physical characteristics
The primary physical characteristics of a proton are as follows:
Mass: 1.6726 × 10-27 kilograms
Charge: +1.6022 × 10-19 coulombs
Radius: approximately 0.87 × 10-15 meters
Spin: 1/2
Magnetic moment: 1.4106 × 10-26 joules/tesla
Lifetime: greater than 1029 years (theoretically unlimited)
In the Standard Model of elementary particles, a proton is classified as a baryon, which means it is a particle with half-integer spin and an integer charge. Protons play a crucial role in nuclear physics and many fields of science and technology, including nuclear energy, medicine, space exploration, and much more.