Radioactivity (from Latin: radio – “emit” and activus – “active”) is the property of certain elements and isotopes that have unstable atomic nuclei and can emit excess energy in the form of particles or electromagnetic radiation.
This property is due to the unstable state of nuclei, which seek to achieve more stable states by emitting excess energy during radioactive decay.

Symbol used to indicate radioactive materials
Radioactivity was discovered in 1896 by the French physicist A. Becquerel. He was studying the relationship between luminescence and the recently discovered X-rays.
Radioactivity can manifest in three forms of radiation:
- Alpha radiation, which is a stream of alpha particles (which are helium nuclei), with low penetrating ability. Alpha particles can be completely stopped by a sheet of paper.
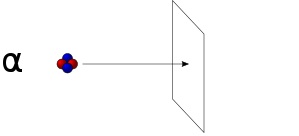
Alpha Radiation
- Beta radiation, which is a stream of beta particles (electrons or positrons), with moderate penetrating ability. Beta particles can be stopped by an aluminum screen.

Beta Radiation
- Gamma radiation, which is a stream of gamma photons, with high penetrating ability. Gamma rays can only be stopped with the use of significantly thicker shielding, such as a thick layer of lead.
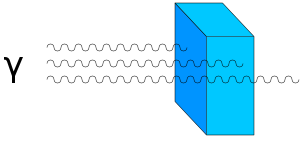
Gamma Radiation
The amount of radioactive isotopes in a substance is measured in Becquerels (Bq), which is equal to one decay per second. Radioactivity can have both beneficial and harmful properties. It is used, for example, in medical research, energy production, and the manufacturing of nuclear weapons. However, a high level of radioactivity can lead to radiation contamination and pose risks to human health and the environment. Therefore, radioactive materials must be used correctly and handled with proper protection.