A semiconductor is a substance that occupies an intermediate position between conductors, which have high electrical conductivity (metals, electrolytes), and dielectrics, which conduct almost no electric current (porcelain, rubber, and others).
The following are considered as semiconductors: metal oxides such as Al2O3, Cu2O, ZnO, TiO2, sulfide compounds such as Cu2S, Ag2S, ZnS, CdS, HgS, selenide compounds, telluride compounds, certain alloys (MgSb2, ZnSb2, AlSb, ClSb), chemical elements such as germanium, silicon, tellurium, boron, carbon, sulfur, phosphorus, arsenic, as well as complex compounds (such as gallium arsenide and silicon carbide).
A semiconductor is a substance with electrical conductivity ranging from 10-10 to 104 (ohm-cm)-1.
The electrical properties of semiconductors are vastly different from conductors and insulators. Electrical conductivity strongly depends on temperature, illumination, the presence and intensity of an electric field, and the amount of impurities.
Types of conductivity
Semiconductors have two types of conductivity: electronic and hole conductivity.
Electronic conductivity is carried out by free electrons, while hole conductivity is due to the movement of bonds that are left without electrons.
Electronic conductivity
Conductivity that is caused by the movement of electrons is called electronic conductivity and is denoted by the letter n (n-type conductivity).
Free electrons move within the crystal lattice of the semiconductor, similar to free electrons in metals. Conductivity of a semiconductor that is due to the movement of electrons is called electronic conductivity.
Hole conductivity
Conductivity that is due to the movement of holes is called hole conductivity and is denoted by the letter p (p-type conductivity).
As a result of the destruction of covalent bonds, a vacant place is immediately formed at the site of each broken bond, which is called a hole. Since the hole in the crystal moves like a free carrier of electric charge, it is attributed a positive charge. If an external electric field is present, an ordered movement of holes occurs in the semiconductor, and a current associated with the movement of holes is added to the current of free electrons.
Intrinsic conductivity of semiconductors
A pure semiconductor has equal degrees of electronic and hole conductivity (in terms of an equal number of carriers of both types).
The conductivity of pure semiconductors without any impurities is called intrinsic conductivity of semiconductors. Intrinsic conductivity of semiconductors is low because of the low number of free electrons.
In a pure germanium crystal and other semiconductor crystals, there are no free electrons at low temperatures and such crystals are good insulators under these conditions.
The conductivity of chemically pure semiconductors becomes possible when the covalent bonds in crystals are broken. For example, heating to relatively low temperatures causes the covalent bonds to break, creating free electrons and causing the appearance of intrinsic electronic conductivity of pure semiconductors (n-type conductivity).
The energy required to create electrical conductivity in pure semiconductor crystals is called the activation energy of intrinsic conductivity.
As the temperature increases, the number of covalent bonds breaking and the number of free electrons in pure semiconductors increases.
This means that the specific electrical conductivity of pure semiconductors increases with temperature, and the specific resistance decreases. This sets semiconductors apart from metals, where the specific resistance increases with heating.
In addition to heating, the breaking of covalent bonds and the emergence of intrinsic conductivity can be caused by illumination (photovoltaic effect) or the action of strong electric fields.
When a crystalline pure semiconductor receives the energy required to break covalent bonds and an electron leaves its place, the electrical neutrality of the crystal at that location is disturbed. At the site from which the electron left, there is an excess positive charge – a positive hole appears, which behaves like a charge that is equal in absolute value to the charge of an electron but positive in sign. An adjacent electron can move to the place vacated by the electron, which is equivalent to the movement of a positive hole: it appears in a new location from where the electron left.
Under the influence of an external electric field, electrons move in the direction opposite to the direction of the electric field intensity. Positive holes move in the direction of the electric field intensity, i.e., in the direction where a positive charge would move under the influence of an electric field. The process of electron and hole movement in an external electric field occurs throughout the semiconductor crystal. The electrical conductivity of a pure semiconductor is determined by the ordered movement of holes and is called intrinsic hole conductivity (p-type conductivity).
The total specific conductivity of a semiconductor consists of n- and p-type conductivity.
Extrinsic conductivity of semiconductors
If a small percentage of impurities is added to a pure conductor, the mechanism of conductivity will change. Impurities are added to increase the number of charge carriers. With an increase in the number of charge carriers, the electrical conductivity increases. In the presence of impurities, in addition to the intrinsic conductivity, there is also extrinsic conductivity. By changing the concentration of impurities, it is possible to change the number of charge carriers of one sign or another, thereby creating semiconductors with a predominance of positively or negatively charged carriers.
Donor dopant (n-type semiconductor conductivity)
Semiconductors with donor dopants have n-type conductivity.
If a five-valent arsenic (As) is added to four-valent germanium (Ge), the dopant atoms will take the place of germanium atoms in the crystal lattice. The fifth electron will become surplus and easily detach from the atom, becoming free. This leads to an increase in electrical conductivity. When an external electric field is applied to a semiconductor, the movement of charges becomes directed. A dopant with a valency that exceeds the valency of the host atoms is called a donor dopant.
In a crystal of germanium with arsenic dopant, there are electrons and holes that create the crystal’s intrinsic conductivity. However, the main type of free charge carriers is electrons detached from arsenic atoms. This type of conductivity is called electron conductivity, and a semiconductor that has electron conductivity is called an n-type semiconductor.
Acceptor impurity (p-type semiconductor)
P-type conductivity is exhibited by semiconductors with acceptor impurities.
If we add a trivalent indium (In) instead of arsenic to four-valent germanium, it lacks one electron to form a covalent bond, leaving a positively charged electron vacancy or “hole.” When an external electric field is applied, the movement of electrons becomes directional, and electrons from neighboring atoms move from one hole to another.
р—n – transition
The contact between two semiconductors with different conductivity types is called a p-n junction.
It is impossible to create a junction simply by touching plates of n- and p-type, since this would inevitably result in an intermediate layer of air, oxides, or surface contaminants. These transitions are obtained by melting or diffusing appropriate impurities into single crystal plates of semiconductor, as well as by growing p-n junctions from a melt of a semiconductor with a controlled amount of impurities.
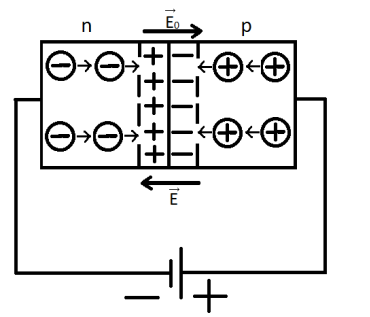
Diffusion is the process of mutual penetration of molecules or atoms of one substance between molecules or atoms of another substance, which usually leads to the alignment of their concentrations throughout the occupied volume.If a piece of indium is melted onto a phosphorus-doped silicon crystal, then some of the indium atoms will diffuse into the crystal and form regions with a different type of conductivity.In the contact between p-type and n-type semiconductors, there is mutual diffusion of electrons and holes and their neutralization, resulting in the formation of a depletion layer that has a high resistance. In the depletion layer, an electric field E0 is created, directed from n to p, and a contact potential difference (potential barrier) is formed, which limits further diffusion of charge carriers.
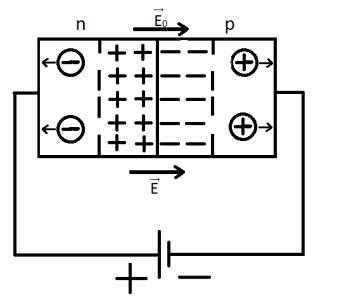
Direct connection of p-n junction – transition.
If an E field is created directed from the p- to n-type semiconductor, the resistance of the depletion layer will decrease and a forward current will begin to flow through the p-n junction. The resistance of the junction decreases with this type of connection, and the width of the depletion layer decreases. With this type of connection, the p-n junction is open.
Reverse connection of p-n junction – transition.
If an E field is created directed from the n- to p-type semiconductor, the direction of the electric field coincides with the internal electric field. The resistance increases, and the depletion layer widens.
The ability to allow current to flow in only one direction is called the rectifying effect or rectification, not the vental effect.
The Volt-Ampere characteristics of a p-n junction.
Direct branch (direct connection)
- A – The direct electric field is less than the blocking field and the current passing through the p-n junction is insignificant.
- B – As the direct voltage increases, the p-n junction opens up.
Reverse branch (reverse connection)
- D – When the reverse bias is applied, the potential barrier for charge carriers increases. The resulting current increases but will remain constant because the number of minority carriers is limited, and all of them participate in the current transport mechanism – “saturation”.
- E – Further increase in the reverse voltage leads to an increase in the velocity of charge carriers, and their kinetic energy reaches the magnitude of impact ionization of atoms in the p-n junction. As a result, the number of electrons and holes in the p-n junction increases avalanche-like, leading to an increase in current at a constant voltage – this is called the avalanche breakdown of the p-n junction. It has a reverse character
Further increase in current leads to heating up of the crystal, which causes generation of additional charges and leads to thermal breakdown of the p-n junction.
- F – Due to the breakdown of the p-n junction, it is destroyed.
Semiconductor Applications
Semiconductors have found the widest range of applications in modern technology, and have had a very strong impact on technical progress. They have made it possible to significantly reduce the weight and size of electronic devices. The development of all areas of electronics has led to the creation and improvement of a large amount of various equipment using semiconductor devices.
However, some semiconductor materials have a low temperature limit (for example, germanium), but this disadvantage can be overcome by using simple temperature compensation or by replacing the main material of the device with another material (such as silicon or silicon carbide).
Semiconductors are used in the production of semiconductor diodes, double transitions (p-n-p or n-p-n) transistors and thyristors. These devices are mainly used for rectification, generation, and amplification of electrical signals. Based on the photoelectric properties of semiconductors, photoresistors, photodiodes, and phototransistors are created. Semiconductors serve as the active part of the oscillation generator (amplifier) of semiconductor lasers. When an electric current is passed through the p-n junction in the forward direction, charge carriers – electrons and holes – recombine with the emission of photons, which is used in the creation of light-emitting diodes, which are used in lighting devices such as LED lamps and projectors.
Semiconductor devices have gained widespread use in the world, revolutionizing electronics. They serve as the basis for the development and production of measurement equipment, computers, equipment for all types of communication and transport, for process automation in industry, scientific research equipment, rocket technology, medical equipment, and other electronic devices and instruments. The use of semiconductor devices allows for the creation of new equipment and improvement of old equipment, leading to a significant reduction in size, weight, power consumption, and heat dissipation in the circuit, as well as an increase in strength, and immediate readiness for… (the rest of the sentence is incomplete).