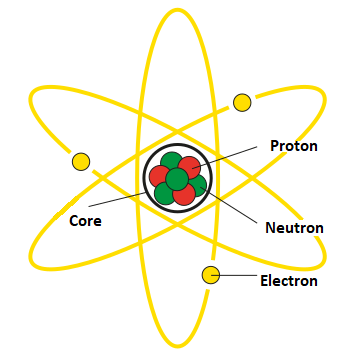
Neutron – is an elementary particle with no electric charge that is part of the atomic nucleus.
History of Neutron Discovery
The discovery of the neutron was a significant event in the history of science. Before that time, two main particles of the atom were known: electrons, which have a negative charge and move around the nucleus, and protons, which have a positive charge and form the nucleus. Considering the interaction of protons with electrons, the atom would be unstable, as electrons should move in a circle around the nucleus and emit energy. However, it was known that atoms are stable and exist for a long time. Therefore, in 1930, the existence of another neutral particle that holds the nucleus together was proposed.
In 1932, James Chadwick commissioned Milton Hamilton to conduct an experiment to detect this new particle. Hamilton used high-energy protons fired from a gun to bombard beryllium nuclei. After this, beryllium nuclei were ejected into the detector zone, which aimed to detect secondary particles released during nuclear decay. After several months of work, Hamilton and his colleague John Kromschroeder detected the new neutral particle, which they named the neutron.
Physical Properties and Structure of Neutron
The neutron is an elementary particle with no electric charge. It has a mass approximately equal to the mass of a proton, and its size is slightly larger than that of a proton. In fact, neutrons and protons have very similar physical properties, except that the neutron lacks an electric charge.
The neutron consists of three fundamental particles: two long quarks and one short quark. Quarks are elementary particles that make up protons and neutrons. The long quarks, named “up” and “down,” have an electric charge, while the short quark, called “strange,” has no electric charge.

Although the neutron has no electric charge, it has a magnetic moment. The neutron’s magnetic moment reflects how quickly it rotates around its axis. One of the key properties of the neutron is its interaction with other particles. If a neutron collides with a proton, they can form a nucleus. If a neutron collides with another neutron, nuclear decay can occur.
There are two types of neutrons – light and heavy. Light neutrons have a mass of approximately 1.008665 daltons, while heavy neutrons have a mass of approximately 1.009187 daltons. Light neutrons are more common in nature, while heavy neutrons arise from nuclear reactions.
Another important characteristic of the neutron is its half-life. The half-life is the time it takes for half of a certain quantity of radioactive particles to decay. In the case of neutrons, the half-life is very short, about 10 minutes.
Neutrons can exist in different energy states, which is reflected in their spectrum. According to the laws of quantum mechanics, the neutron’s energy state can be represented as a combination of different spin and angular momentum values.
Neutrons also have the property of absorbing neutrons. This means that if a neutron interacts with another neutron, it can increase its energy and exit the nucleus, leading to nuclear fission and the emergence of a chain reaction. This process is used in nuclear reactors in nuclear power plants and nuclear bombs.
Neutrons also have the property of scattering on nuclei, which can be used to study nuclear properties. For this purpose, neutrons are subjected to scattering on a sample and then analyzed using detectors.
Another property of neutrons is their interaction with various materials. Neutrons can be slowed down in different materials, which can be used for control over nuclear reactions and applied in various industries.
Conclusion
This article discusses the history of neutron discovery, its physical properties, and structure.
A neutron is a fundamental particle that has no charge but has mass, magnetic moment, and other properties.
Neutrons have important applications in nuclear physics, medicine, industry, and other fields.
For example, neutron sources can be used for industrial control and material diagnostics.
Neutrons are also used in cancer treatment and other medical applications.