Diffusion is the process of mutual penetration of molecules or atoms of one substance among the molecules or atoms of another, which typically results in the equalization of their concentrations throughout the occupied volume. Diffusion occurs in gases, liquids, solids, and plasmas.
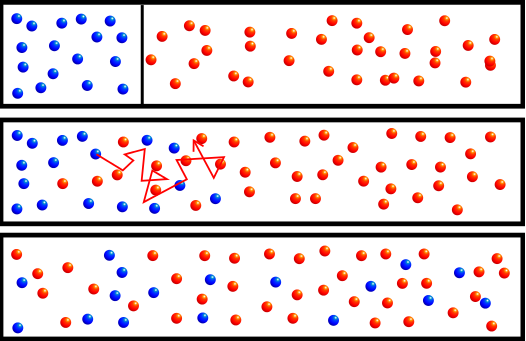
The fundamental principles of diffusion include the following:
- Random motion: Particles move randomly and chaotically due to their thermal vibrations.
- Concentration gradient: Diffusion occurs from areas of higher concentration to areas of lower concentration, aiming to equalize the concentration gradient.
- Time: The rate of diffusion depends on time. The longer the time elapsed, the more particles will be distributed throughout the system.
- Cross-sectional area: The plane through which diffusion takes place affects the rate of diffusion. The larger the cross-sectional area, the more particles can pass through it per unit time.
Types of diffusion
The main types of diffusion include:
- Gas diffusion: This is the spreading of gas particles within a gaseous medium. Gases move randomly and fill the available space. This type of diffusion describes phenomena like the spread of odor in the air.
- Liquid diffusion: In liquids, diffusion occurs through the movement of substance molecules. The most well-known example is the diffusion of dissolved substances in a liquid. This process plays a significant role in biological systems, such as respiration, cellular activity, and substance transport in the body.
- Solid diffusion: Diffusion in solids takes place through the movement of atoms or molecules within the internal structure of a solid. This process can occur at elevated temperatures and is crucial for processes like diffusion strengthening of metals or the diffusion of impurities in semiconductors.
- Membrane diffusion: Membranes, such as cell membranes, can allow certain substances to pass through, creating a concentration gradient. This type of diffusion is important for regulating substances and ions within cells.
- Mass diffusion: This is the diffusion of substances in multi-component systems, where the movement of substances occurs from regions of higher concentration to regions of lower concentration. This process is used in chemical reactions where substances react with each other in a solution.
These types of diffusion can coexist in different systems and have significant implications for understanding physical, chemical, and biological processes occurring in nature and industry.
History
Diffusion is a phenomenon observed in nature over an extended period, and it has been studied by humanity throughout the history of scientific research.
Here are a few notable moments in the history of diffusion research:
- Early observations: People began observing the phenomenon of diffusion long before it was systematically studied. For instance, observations of the diffusion of scents from aromatic substances spreading in the air were known since ancient times.
- Development of mathematical models: In the 19th century, physicists began developing mathematical models to describe diffusion. French scientist Adolphe Fick formulated Fick’s law in 1855, which describes the spread of substances in a medium.
- Brownian motion studies: In 1827, botanist Robert Brown observed irregular movements of pollen particles in a liquid, which were related to the molecular motion of the substance. These observations, known as “Brownian motion,” are a significant discovery in the study of diffusion.
- Kinetic theory of gases: In the 19th century, physicists like Ludwig Boltzmann and James Clerk Maxwell developed the kinetic theory of gases, which explains the molecular motion of gases and includes the concept of diffusion.
- Modern research: With the advancement of modern science and technology, diffusion research has become more accurate and detailed. Contemporary methods, such as computer modeling and experiments using microscopes, allow researchers to study diffusion at the molecular level and apply it in various scientific and practical fields.
A common trend in the history of diffusion research is the gradual expansion of knowledge about this phenomenon, the development of mathematical models, and the increased precision of experimental methods. Diffusion remains an important subject of study in science and holds significance in various fields, including physics, chemistry, biology, materials science, and engineering.