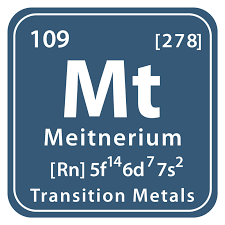
Meitnerium is a chemical element with the symbol Mt and atomic number 109. It is a synthetic element that does not occur naturally.
It was first synthesized in 1982 by bombarding 209Bi nuclei with 58Fe nuclei through the reaction 209Bi + 58Fe → 266Mt + n at the UNILAC accelerator by a group of German scientists led by Peter Armbruster and Gottfried Münzenberg at the Heavy Ion Research Center in Darmstadt, Germany.
Meitnerium is named after the Austrian-Swedish physicist Lise Meitner, who made significant contributions to the development of nuclear physics. She was one of the first scientists to suggest the possibility of nuclear fission.
Mt belongs to the 9th group of the periodic table of chemical elements and is located in the seventh period of the table.
Meitnerium is a radioactive element with a very short half-life. The most stable isotope, meitnerium-278, has a half-life of 7.6 seconds. Mt decays into smaller atoms such as dubnium and seaborgium.
Mt is a highly rare element. It has only been synthesized in very small quantities in the laboratory and has no practical applications.
Chemical Properties of Meitnerium
The chemical properties of meitnerium are insufficiently studied due to its short half-life and the complexity of its synthesis. However, based on its position in the periodic table and the chemistry of its homologs, some assumptions can be made about its chemical properties.
- Oxidation States: It is expected that Mt will demonstrate oxidation states of +3, +4, +5, +6, and +7.
- Electronegativity: Mt is expected to be less electronegative than its platinum homolog but more electronegative than its rhenium homolog.
- Solubility: Mt is likely to dissolve in aqua regia and concentrated sulfuric acid.
- Complex Formation: Mt may form complexes with various ligands such as fluorine, chlorine, bromine, iodine, and ammonia.
- Redox Reactions: Mt may participate in redox reactions as both an oxidant and a reductant.
Some possible chemical compounds of meitnerium:
- MtCl3 (meitnerium(III) chloride)
- MtO2 (meitnerium(IV) oxide)
- MtF5 (meitnerium(V) fluoride)
- Mt2(SO4)3 (meitnerium(III) sulfate)
- K2MtO4 (meitnerate(VI))
It is important to note that the above chemical compounds of Mt have not been synthesized and experimentally confirmed. Their existence is based on theoretical calculations and the chemistry of meitnerium’s homologs.
Physical Properties of Meitnerium
The physical properties of meitnerium are insufficiently studied due to its short half-life and the complexity of its synthesis. However, based on its position in the periodic table and the physics of its homologs, some assumptions can be made about its physical properties.
- Atomic Mass: The atomic mass of meitnerium is [278].
- Melting Point: It is expected that Mt will have a high melting point, similar to its platinum and rhenium homologs.
- Boiling Point: Mt is expected to have a high boiling point, similar to its platinum and rhenium homologs.
- Density: It is expected that Mt will have a high density, similar to its platinum and rhenium homologs.
- Thermal Conductivity: Mt is expected to have high thermal conductivity, similar to its platinum and rhenium homologs.
- Electrical Conductivity: It is expected that Mt will have high electrical conductivity, similar to its platinum and rhenium homologs.
Some possible physical properties of Mt:
- Melting Point: 2000-2500 °C
- Boiling Point: 4000-4500 °C
- Density: 20-25 g/cm3
- Thermal Conductivity: 100-150 W/(m·K)
- Electrical Conductivity: 106-107 S/m
It is important to note that the above physical properties of meitnerium have not been experimentally confirmed. Their values are based on theoretical calculations and the physics of meitnerium’s homologs.
Isotopes
| Isotope | Mass | Half-life | Decay Mode |
|---|---|---|---|
| 266Mt | 266 | 1.7+1.8−1.6 ms | α-decay to 262Bh |
| 268Mt | 268 | 21+8−5 ms | α-decay to 264Bh |
| 270Mt | 270 | 5.0+2.4−0.3 ms | α-decay to 266Bh |
| 275Mt | 275 | 9.7+46.0−4.4 ms | α-decay to 271Bh |
| 276Mt | 276 | 0.72+0.87−0.25 s | α-decay to 272Bh |