Methanol, also known as methyl alcohol or wood alcohol, is a chemical compound with the formula CH3OH. It is one of the simplest alcohols and has a wide range of applications in various industries and scientific fields.
Methanol Formula
Molecular formula of methanol:
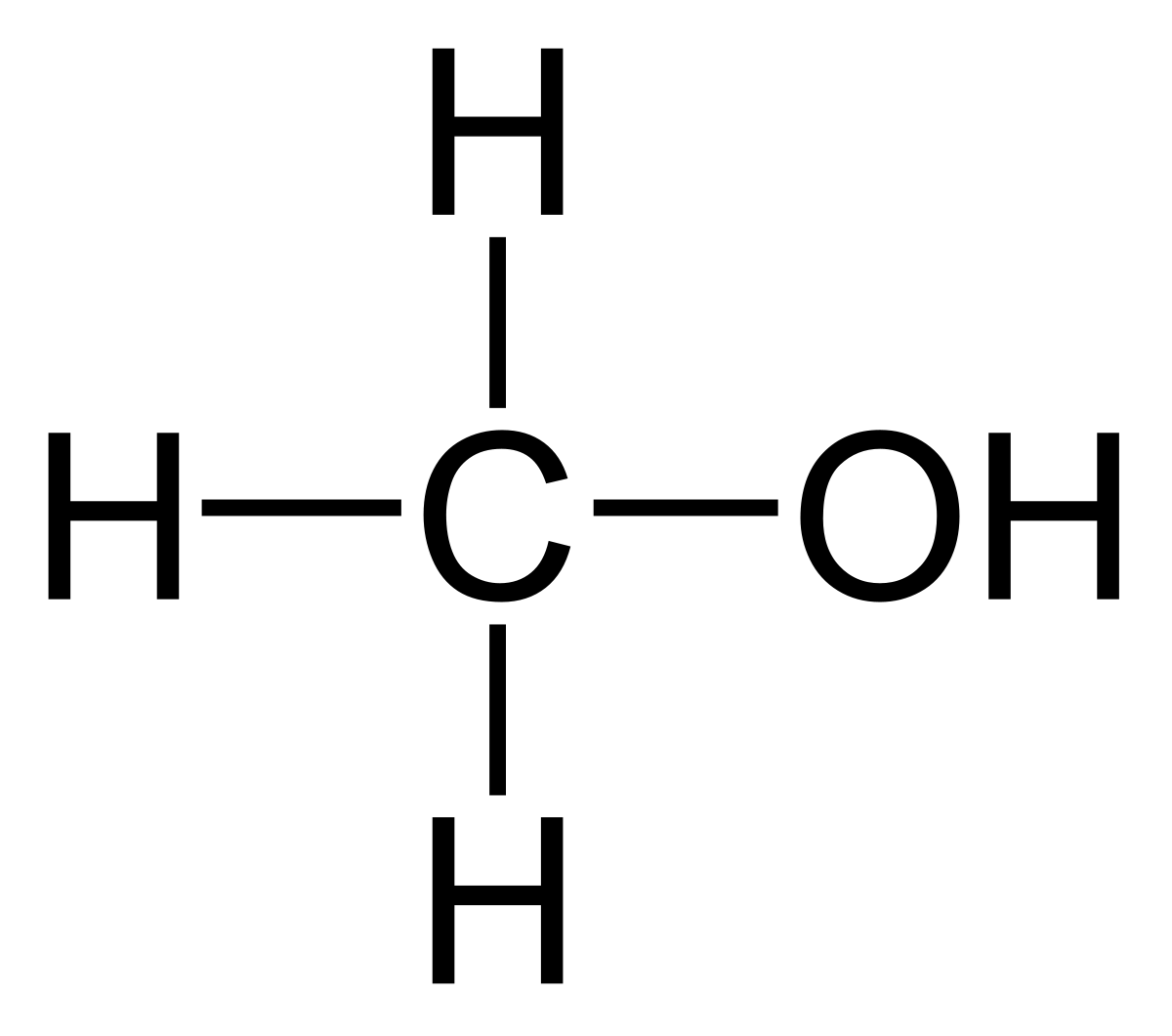
CH3OH
This substance consists of one carbon atom (C), one oxygen atom (O), and three hydrogen atoms (H) in its molecule.
Structural formula of methanol:
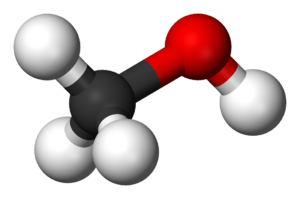
Ball-and-stick model of CH3OH:
History of Discovery and Applications
The history of methanol encompasses numerous significant discoveries and applications over many centuries.
Discovery and Extraction
Methanol was first identified as a distinct substance in the 17th century. Its extraction from wood was achieved by the distillation of wood, giving rise to the names “wood spirit” or “wood methyl alcohol.” Oak wood was a primary source.
The Name “Methanol
“The term “methyl alcohol” was first proposed by scientist Carl Séras in 1661. In 1834, Joseph Gay-Lussac introduced the term “methanol,” derived from the Greek word “methy,” meaning “intoxication,” and “alcohol,” indicating its alcoholic nature.Application as FuelIn the 19th century, CH3OH was used as the primary raw material for gas lighting.
Synthesis and Production
Various methods of CH3OH synthesis, including the catalytic conversion of hydrocarbons and the oxidation of methane, were developed in the 20th century. Industrial production of methanol evolved from this point.
Physical Properties of Methanol
- CH3OH is a colorless, volatile liquid with a distinctive odor.
- The melting point of CH3OH is approximately -97.6 °C, and its boiling point is around 64.7 °C.
- CH3OH is a solvent for many substances and readily dissolves in water.
Chemical Properties of Methanol
- CH3OH is an alcohol, featuring a hydroxyl (-OH) functional group that makes it reactive with many chemical compounds.
- A crucial reaction is the oxidation of CH3OH to formaldehyde (HCHO) or acetic acid (CH3COOH).
- CH3OH can be used as a raw material for the synthesis of various chemical compounds, including formaldehyde, acetic acid, formaldehyde resin (methanal), and others.
Applications of CH3OH
- Production of formaldehyde, used in the chemical industry for manufacturing plastics, paints, varnishes, and lacquer products.
- Manufacturing acetic acid, utilized in the food industry and the production of various chemical products.
- CH3OH can be used as a fuel, usually in blends with gasoline, hydrogen, or other fuels, referred to as “methanol-based fuels.”
- Production of formaldehyde resin (methanal), used in the synthesis of plastics and paints.In laboratory practice, CH3OH is used for dissolving various chemical compounds and as a reagent in chemical experiments.
It is important to note that CH3OH is toxic to humans and animals, so appropriate safety measures should be taken when working with it.