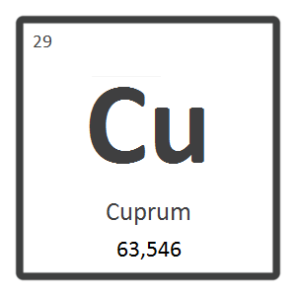
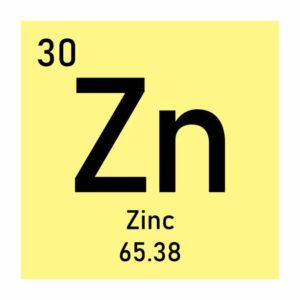
Properties of magnesium
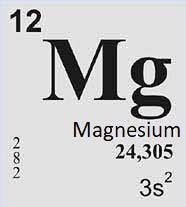
Magnesium is a chemical element with the symbol Mg and atomic number 12. It is a silvery-white metal that belongs to the alkaline earth metals group in the periodic table. Physical properties of magnesium Here are some physical properties of magnesium: Overall, the physical properties of magnesium make it a useful metal in various industrial …