Category «Elements»
Sulfuric acid

Sulfuric acid (H2SO4) is a colorless, viscous liquid and is one of the most important chemical substances. It is a strong acid with high reactivity and is widely used in the industry. Also known as: sulphuric acid. Chemical properties of sulfuric acid It’s important to note that H2SO4 is highly corrosive and dangerous, requiring careful …
Hydrochloric acid
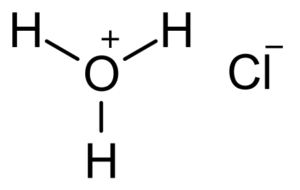
Hydrochloric acid (HCl) is a chemical compound composed of chlorine (Cl) and hydrogen (H). Other names: hydrogen chloride, muriatic acid. It has the chemical formula HCl and is one of the most common and essential acidic compounds. Hydrochloric acid is highly acidic and finds widespread use in various industrial and laboratory processes. It has a …
Xenon
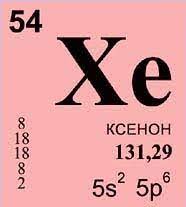
Xenon (Xe) is a chemical element that belongs to the noble gases in the periodic table. Here are some general facts about xenon: Physical Properties of Xenon The main physical properties of Xe include the following: These physical properties of Xe make it useful in various applications. Chemical Properties of Xenon Chemical properties of Xe …
Properties of hydrogen
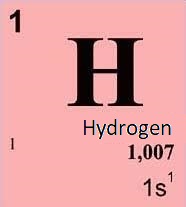
Hydrogen is a chemical element with the symbol H and atomic number 1. It is the lightest element in the periodic table and is also the most abundant element in the universe, accounting for around 75% of its elemental mass. Physical properties of hydrogen Chemical properties of hydrogen Other properties
Properties of gold

Gold is a chemical element with the symbol Au and atomic number 79. Physical properties of gold Chemical properties gold Other Properties gold Іsotopes of gold Gold has one stable isotope, which is 197Au, meaning it has 197 nucleons (protons and neutrons) in its nucleus. However, there are several radioactive isotopes of gold that have …
Properties of francium

Francium is an extremely rare and unstable metal with the atomic number 87 and symbol Fr. Because it is so rare and unstable, only a very small amount of information is known about its properties. Alkali metal. Physical properties of francium Due to its rarity and instability, very few physical properties of francium have been …
Properties of cesium

Cesium is a highly reactive alkali metal with the atomic number 55 and symbol Cs. Physical properties of cesium Here are some of its physical properties: Overall, cesium has unique physical properties, such as its low melting point, low boiling point, and high reactivity, that make it a valuable element for various applications in science …
Properties of rubidium

Rubidium is a chemical element with the symbol Rb and atomic number 37. It is a soft, silvery-white metallic element that belongs to the alkali metal group of elements. Physical properties of rubidium Rubidium is a soft, silvery-white metal with the atomic number 37 and symbol Rb. Here are some of its physical properties: Overall, …
Properties of potassium

Potassium is a chemical element with the symbol K and atomic number 19. It is a highly reactive alkali metal that is essential for many biological processes. Physical properties of potassium Some physical properties of potassium: Overall, the physical properties of potassium make it a unique and important element in various industries and biological processes. …
